In a landmark decision that could reshape the landscape of mental health treatment, the Food and Drug Administration (FDA) has approved Johnson & Johnson’s Spravato as a standalone treatment for major depressive disorder (MDD). This ketamine-derived nasal spray represents a significant breakthrough for millions of Americans who have struggled to find relief through traditional antidepressant medications.
A New Hope for Treatment-Resistant Depression
The approval of Spravato as a monotherapy marks a pivotal moment in psychiatric medicine. For the first time, adults living with major depressive disorder who haven’t responded adequately to at least two oral antidepressants have access to a fundamentally different treatment approach. This development is particularly crucial given the staggering statistics surrounding depression in the United States.
Understanding the Impact
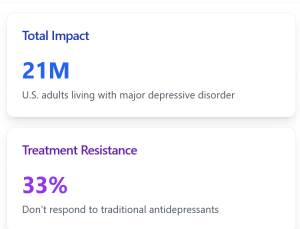
Major depressive disorder affects approximately 21 million adults in the United States, making it one of the most prevalent psychiatric conditions. What makes this approval particularly significant is its potential to help the estimated one-third of patients who don’t respond to conventional oral antidepressants. These individuals, who suffer from what’s known as treatment-resistant depression (TRD), have historically had limited options for managing their condition.
Revolutionary Treatment Timeline
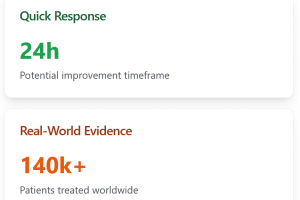
One of the most remarkable aspects of Spravato is its rapid onset of action. Unlike traditional antidepressants, which often take weeks to show effectiveness, patients using Spravato may experience symptom improvement as early as 24 hours after administration. This quick response time could be life-changing for individuals in acute depressive episodes.
Backed by Extensive Research
The FDA’s approval wasn’t granted lightly. It follows:
More than a decade of comprehensive research
Almost six years of real-world evidence collection
Successful treatment of over 140,000 patients worldwide
The Ketamine Connection
The Science Behind Ketamine-Based Treatment
Ketamine’s journey from operating rooms to psychiatric clinics represents one of the most intriguing developments in modern medicine. Originally known as a dissociative anesthetic, ketamine has emerged as a promising tool in mental health treatment. The drug works differently from traditional antidepressants, which typically target serotonin, norepinephrine, or dopamine systems. Instead, ketamine appears to influence glutamate, the brain’s primary excitatory neurotransmitter, potentially leading to rapid changes in brain chemistry and neural connections.
Growing Public Awareness and Discussion
The treatment has gained increased attention recently, partly due to high-profile figures discussing their experiences with ketamine therapy. While these discussions have helped reduce stigma around mental health treatment, they’ve also highlighted the importance of distinguishing between FDA-approved medical uses and off-label applications.
Important Safety Considerations
As with any significant medical advancement, proper context and caution are essential:
- Medical Supervision: Spravato is designed for administration under medical supervision, ensuring both safety and optimal therapeutic benefit.
- Structured Treatment Protocol: The approval comes with specific guidelines for administration and monitoring, differentiating it from off-label ketamine use.
- Individual Assessment: Healthcare providers must carefully evaluate each patient’s suitability for the treatment, considering their complete medical history and previous treatment responses.
Looking to the Future
The approval of Spravato as a standalone treatment represents more than just a new medication option – it signals a potential paradigm shift in how we approach treatment-resistant depression. Bill Martin, Global Therapeutic Area Head of Neuroscience at Johnson & Johnson Innovative Medicine, emphasizes that this development offers hope to patients who have struggled with limited treatment options.
Clinical Implications
Healthcare providers now have an additional tool in their therapeutic arsenal, particularly valuable for patients who:
- Haven’t responded adequately to traditional antidepressants
- Need rapid symptom relief
- Have difficulty maintaining a daily oral medication regimen
Balanced Perspective
While the FDA approval of Spravato as a monotherapy marks a significant advancement, it’s important to maintain a balanced view. The Yale School of Medicine’s report on the increase in off-label ketamine use reminds us of the importance of following established medical protocols and avoiding unsupervised experimentation with similar compounds.
Conclusion
The FDA’s approval of Spravato as a standalone treatment for major depressive disorder represents a significant milestone in psychiatric medicine. While it’s not a universal solution, it offers new hope for millions of Americans who have struggled to find relief through conventional treatments. As our understanding of depression and its treatment continues to evolve, innovations like Spravato pave the way for more personalized and effective approaches to mental health care.
For those interested in learning more about this treatment option, consulting with mental health professionals who can provide personalized guidance based on individual medical history and needs is essential.