A closer look at a groundbreaking clinical trial that could transform emergency pain management protocols*
Introduction
Emergency medical providers face critical decisions every day about how to manage acute pain in trauma patients. The Prehospital Analgesia INtervention (PAIN) trial represents a significant advancement in the quest to determine the most effective and safest pain management strategies for trauma patients experiencing compensated shock.
This Department of Defense-sponsored multicenter study is comparing two medications commonly used for pain management: ketamine hydrochloride and fentanyl citrate. The results could potentially reshape emergency medicine protocols nationwide and improve outcomes for trauma patients.
Study Overview
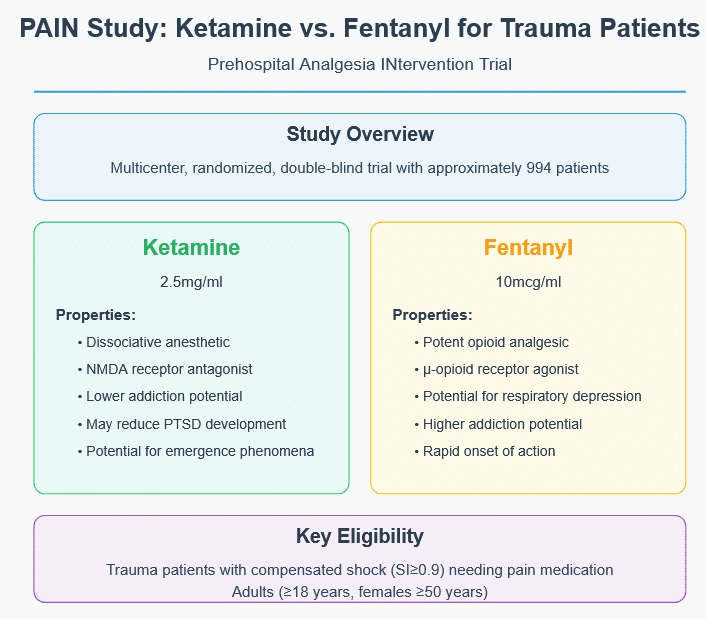
The PAIN study is a 4-year multicenter, randomized, double-blind clinical trial enrolling approximately 994 patients across multiple trauma centers in the United States. Currently, participating centers include:
– Cooper University Health Care (Camden, NJ)
– Allegheny Health Network’s Allegheny General Hospital (Pittsburgh, PA)
– University of Pittsburgh (Pittsburgh, PA)
– Medical College of Wisconsin (Milwaukee, WI – currently suspended)
– University of Vermont Medical Center (Burlington, VT)
As Dr. Dan Wolfson from UVM Medical Center explained, “It reduces long-term negative outcomes, like chronic pain or PTSD and even mortality, but we want to be able to treat pain in the most effective and safest way.”
The Clinical Question
The study addresses a crucial question in emergency medicine: Is ketamine hydrochloride a safer and more effective alternative to fentanyl citrate for pain management in trauma patients with compensated shock?
Why This Matters
– Opioid Crisis Concerns: Fentanyl is a powerful opioid that, while effective for pain management, carries risks of respiratory depression and potential for dependency.
– Alternative Options: Ketamine, traditionally used as an anesthetic, has gained attention for its analgesic properties with potentially fewer respiratory side effects and lower addiction potential.
– Trauma-Specific Effects: Some research suggests ketamine may offer additional benefits in trauma settings, potentially reducing the development of chronic pain and PTSD.
Study Design and Methodology
The PAIN trial employs rigorous scientific methodology:
– Randomized, Double-Blind Design: Neither patients nor providers know which medication is being administered, eliminating bias in assessment.
– Target Population: Adults (≥18 years, females ≥50 years) with compensated shock (Shock Index ≥0.9) requiring IV pain medication.
– Intervention Protocol: Patients receive either ketamine hydrochloride (2.5mg/ml) or fentanyl citrate (10mcg/ml) in blinded, pre-filled syringes, administered via slow IV push.
– Assessment: Pain levels are assessed every 15 minutes using either the Numeric Rating Scale (NRS) or Critical Care Pain Observational Tool (CPOT).
– Re-dosing: Additional medication may be administered after 15 minutes if pain persists and local protocols permit.
Primary and Secondary Outcomes
The study is examining multiple outcomes to thoroughly compare these medications:
Primary Outcome
– 24-hour mortality (all causes)
Secondary Outcomes
– Incidence of hypoxia and hypotension in the prehospital environment
– Need for airway management
– Pain scores following analgesia administration
– Number of doses needed to achieve pain control
– 24-hour opioid use
– Adverse events (allergic reactions, emergence phenomena, dysphoria, etc.)
– Longer-term outcomes (ventilator-free days, ICU-free days, hospital length of stay)
– Long-term pain, anxiety, and PTSD assessments (at 3 and 6 months)
Ethical Considerations and Patient Choice
An important aspect of the PAIN study is its commitment to patient autonomy. While the study uses an exception from informed consent process (common in emergency medicine research), individuals can opt out by:
– Contacting the research team at 1-800-664-0557
– Emailing PAINStudy@edc.pitt.edu
– Requesting a “NO PAIN Study” bracelet
Additionally, the study protocol includes safeguards:
– Exclusion of vulnerable populations
– Following local protocols for contraindications
– Respecting objections voiced by patients or family members at the scene
Potential Impact on Emergency Medicine
The results of this study could significantly influence emergency medicine practice:
1. Evidence-Based Pain Management: Developing clearer guidelines for medication selection based on patient condition and expected outcomes.
2. Reducing Opioid Exposure: If ketamine proves equally or more effective than fentanyl, it could reduce initial opioid exposure in trauma cases.
3. Long-Term Outcome Improvement: The study’s focus on long-term outcomes (PTSD, chronic pain) acknowledges that acute pain management has effects beyond immediate relief.
4. Military Applications: As a Department of Defense-sponsored study, findings may be particularly relevant for battlefield medicine and military trauma care.
Looking Ahead
The comprehensive nature of the PAIN study means results won’t be available for several years. However, the findings promise to provide valuable insights into optimizing pain management for trauma patients.
As emergency medicine continues to evolve, studies like this represent our commitment to evidence-based practice and finding the safest, most effective treatments for patients in their most vulnerable moments.
Read details about the study at clinical trials dot Gov website here.
—
*Note: This article presents information about an ongoing clinical trial. The final results and conclusions of the study are not yet available. Healthcare providers should continue to follow established protocols and best practices for pain management until definitive research findings are published.*
Vermont’s News Station WCAX did a short report on this, click here to read their news story. A video from that story is embedded from youtube below: